
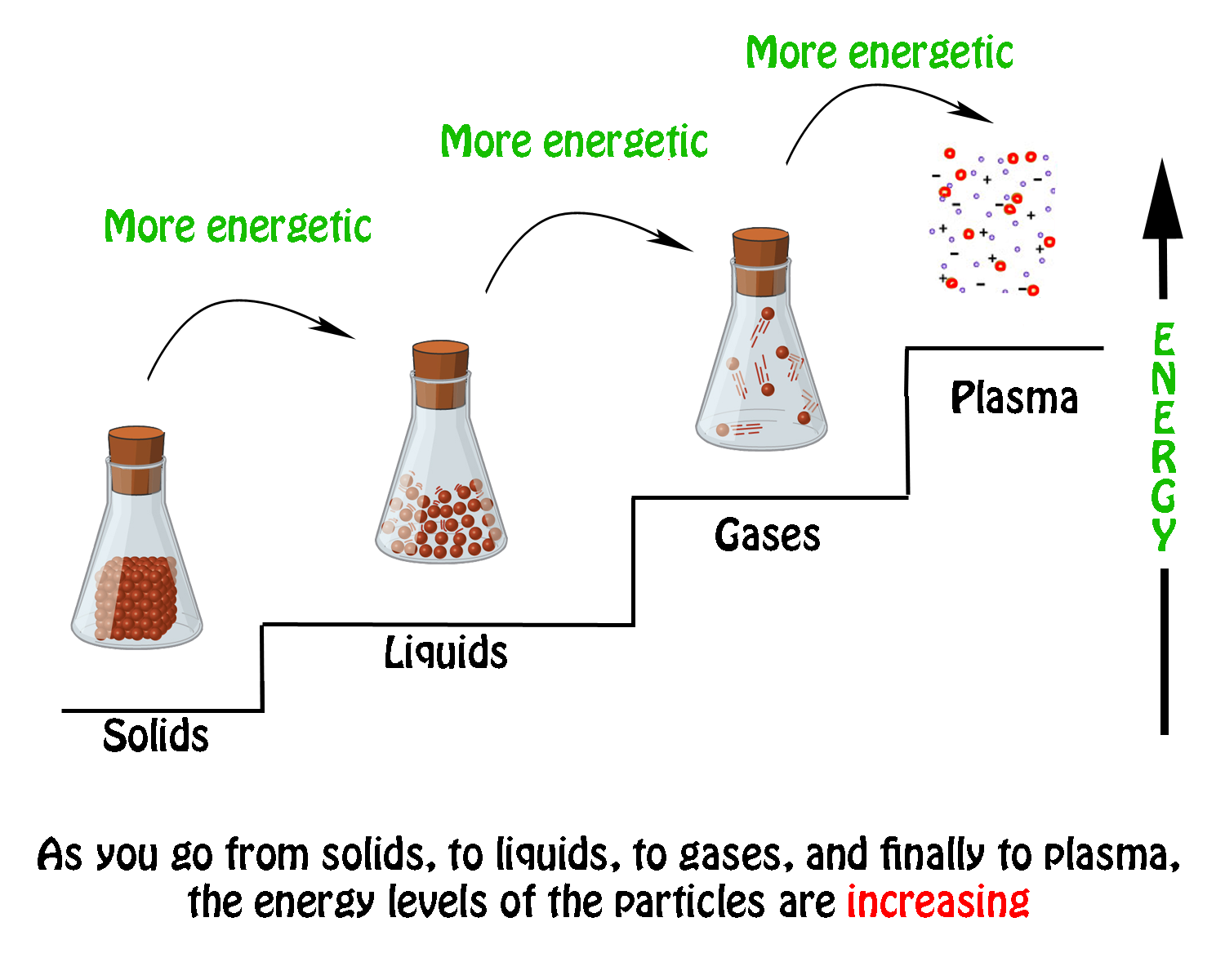
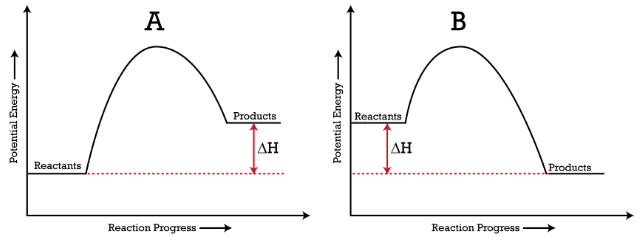
Logic of Exothermic, Endothermic and Entropy: So, Going from a gas to a liquid and liquid to a solid, for example, is going from a less ordered state to a more ordered
Condensation Endothermic Exothermic Ppt Powerpoint Presentation Example File Cpb | Presentation Graphics | Presentation PowerPoint Example | Slide Templates
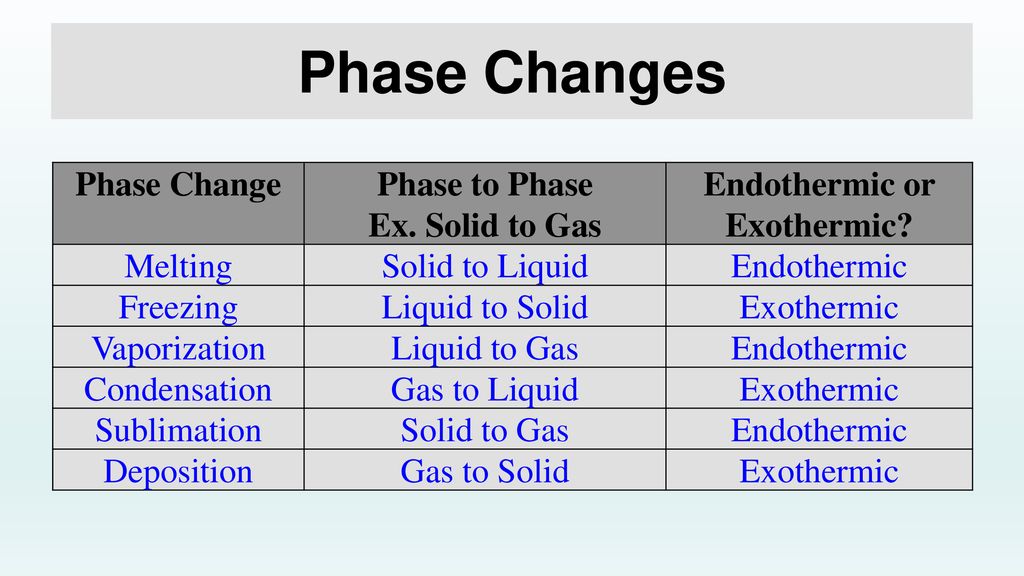
Changes of State Melting, Freezing, Vaporization, Evaporation, Condensation, Sublimation, Deposition OH MY! - ppt download
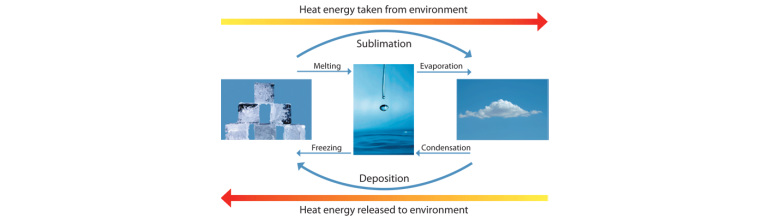
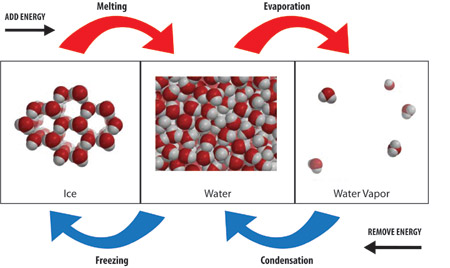
For the three states of matter (solid, liquid, and gas) there are six possible changes of state. Which changes of state are exothermic and which are endothermic ? | Socratic





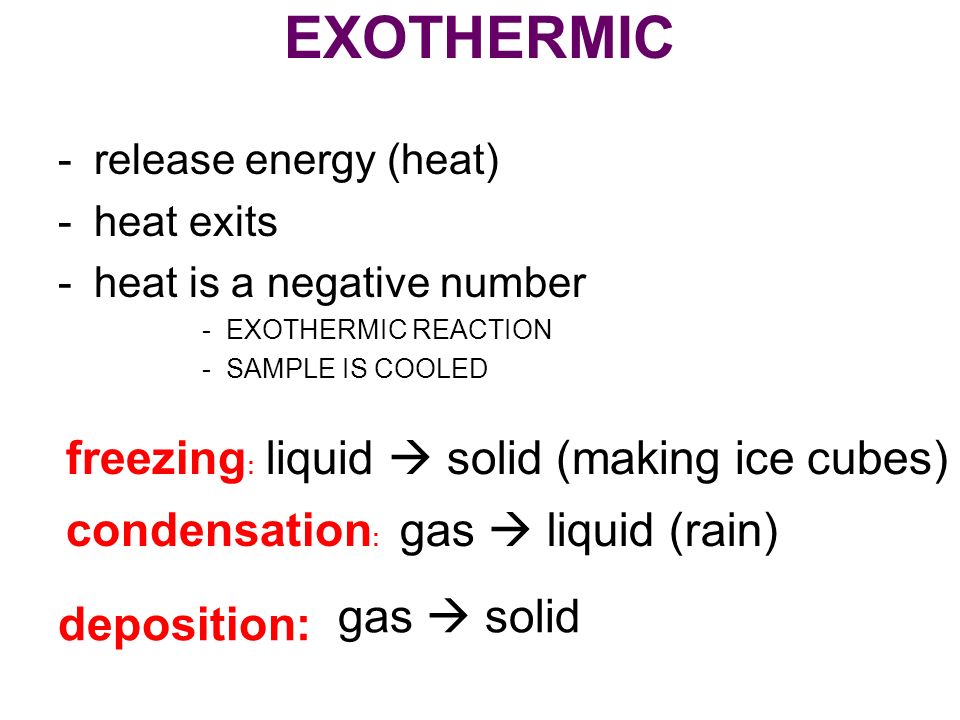








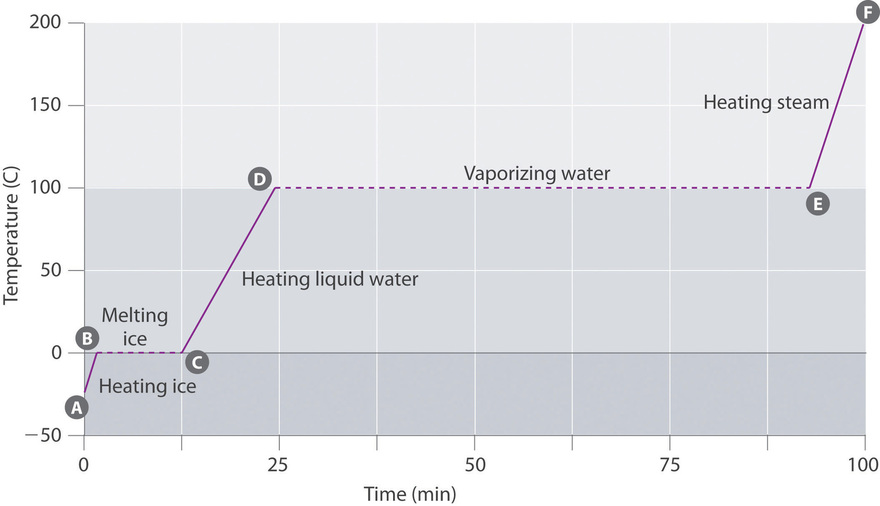

:max_bytes(150000):strip_icc()/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)